← Adherence To The Three-component Hepatitis B Virus Vaccination Protocol Among Healthcare Workers In Hepatitis B Virus Endemic Settings In Ghana Phenotypical Screening Of An MMV Open Box Library And Identification Of Compounds With Antiviral Activity Against St Louis Encephalitis Virus →
Concomitant Administration Of Ad26RSVpreF/RSV PreF Protein Vaccine And High-dose Influenza Vaccine In Adults 65 Years And Older: A Noninferiority Trial
Here is one of the pictures featuring the Concomitant Administration of Ad26RSVpreF/RSV preF Protein Vaccine and High-dose Influenza Vaccine in Adults 65 Years and Older: a Noninferiority Trial. Numerous images associated with the Concomitant Administration of Ad26RSVpreF/RSV preF Protein Vaccine and High-dose Influenza Vaccine in Adults 65 Years and Older: a Noninferiority Trial can be utilized as your reference point. Below, you'll find some extra pictures related to the Concomitant Administration of Ad26RSVpreF/RSV preF Protein Vaccine and High-dose Influenza Vaccine in Adults 65 Years and Older: a Noninferiority Trial.
 Title: Mrna, the beginning of a new influenza vaccine game | pnas
Title: Mrna, the beginning of a new influenza vaccine game | pnasMrna, the beginning of a new influenza vaccine game | pnas.
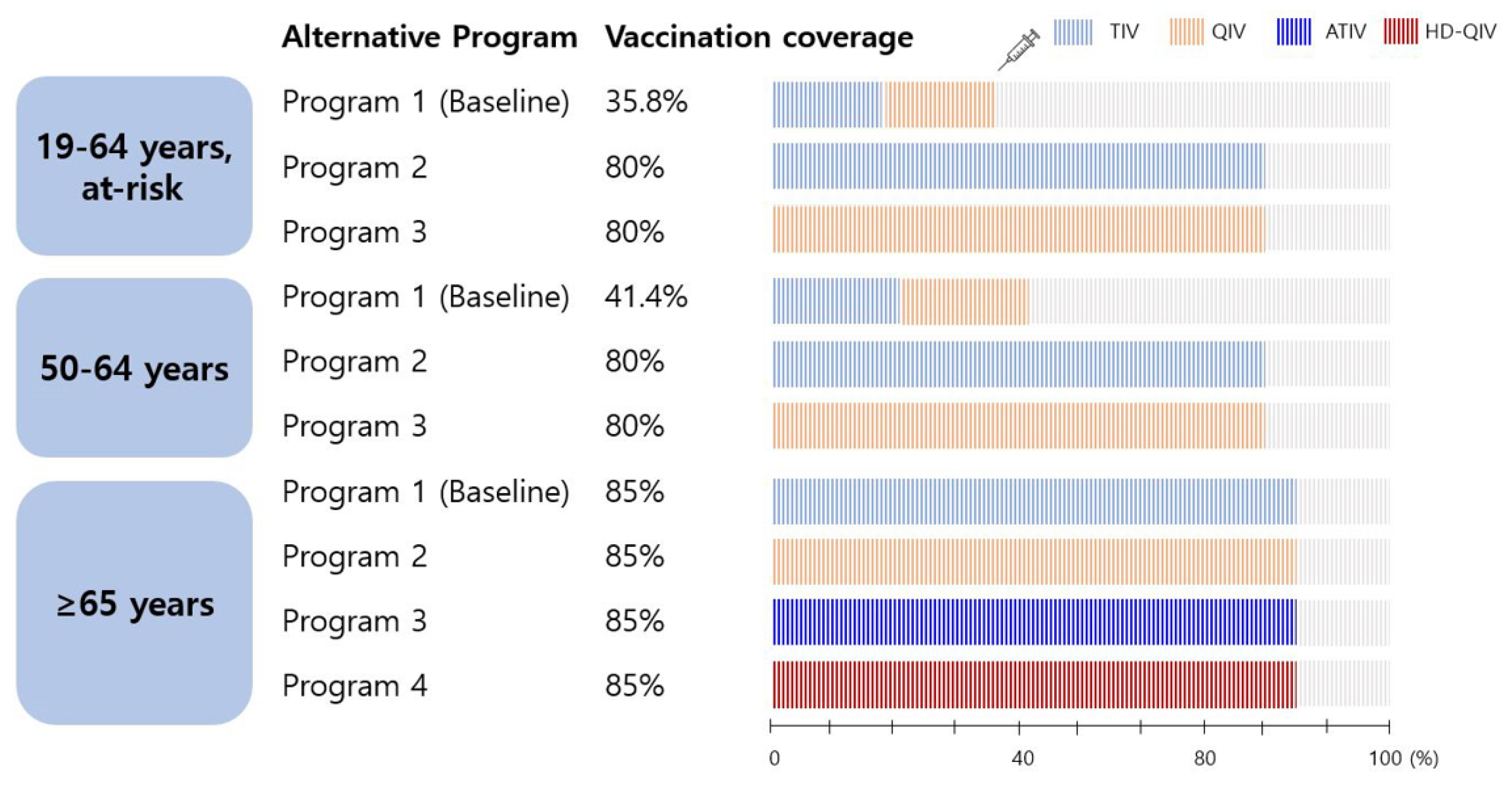 Title: Vaccines | free full-text | cost-effectiveness of influenza vaccination
Title: Vaccines | free full-text | cost-effectiveness of influenza vaccinationVaccines | free full-text | cost-effectiveness of influenza vaccination.
 Title: An rsv vaccine just received fda approval for older adults | the healthy
Title: An rsv vaccine just received fda approval for older adults | the healthyAn rsv vaccine just received fda approval for older adults | the healthy.
