← Self-reported Oral Health Status Augmentative Lag Screws As A Treatment For Aseptic Hypertrophic Nonunion After Internal Fracture Fixation →
Indication And Adverse Event Profiles Of Denosumab And Zoledronic Acid: Based On US FDA Adverse Event Reporting System (FAERS)
Check out one of the pictures featuring the Indication and adverse event profiles of denosumab and zoledronic acid: based on US FDA adverse event reporting system (FAERS). Many images associated with the Indication and adverse event profiles of denosumab and zoledronic acid: based on US FDA adverse event reporting system (FAERS) can be utilized as your reference point. Below, you'll find some more pictures related to the Indication and adverse event profiles of denosumab and zoledronic acid: based on US FDA adverse event reporting system (FAERS).
 Title: Figure 2 from cost-effectiveness of denosumab vs. brand or generic
Title: Figure 2 from cost-effectiveness of denosumab vs. brand or genericFigure 2 from cost-effectiveness of denosumab vs. brand or generic.
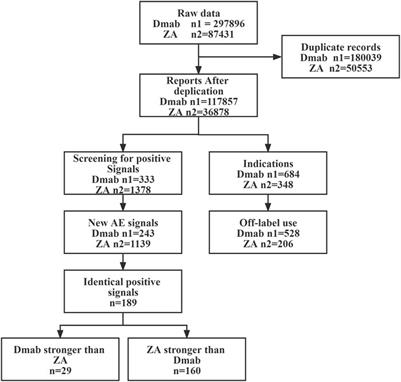 Title: Frontiers | indication and adverse event profiles of denosumab and
Title: Frontiers | indication and adverse event profiles of denosumab andFrontiers | indication and adverse event profiles of denosumab and.
 Title: Adverse event reporting challenges & how to overcome them
Title: Adverse event reporting challenges & how to overcome themAdverse event reporting challenges & how to overcome them.
